Epidemiology of Acute Myocardial Infarction (AMI) and unmet medical needs
What is a heart attack?
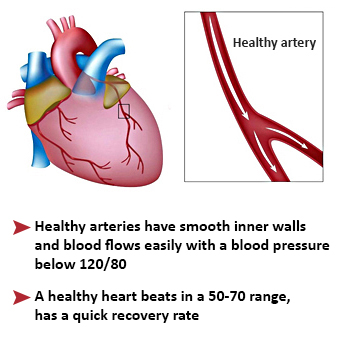
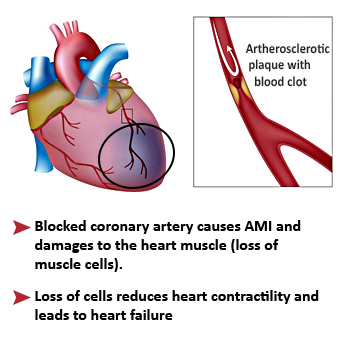
Acute myocardial infarction (AMI), also known as a “heart attack”, is the destruction of an extensive area of the heart muscle. It is mostly the consequence of the obstruction of a coronary artery when cholesterol (fat) accumulates in the form of atheromatous plaques on its walls. A myocardial infarction occurs when an atheromatous plaque breaks off, moves and is trapped in a coronary artery. A blood clot forms around the plaque and interrupts the blood supply, depriving of oxygen the cardiac downstream area which will die.
In France
An average 80 000 people have a heart attack every year, approximately 12 000 of whom will die:
- One person in 10 dies within an hour,
- Then the mortality rate is 15% in the first year. However this rate is progressively decreasing for the past years thanks to the speed and quality of intervention by rescue teams (provided they are called quickly), progress in emergency therapies, and increased interventional cardiology units availability and efficacy.
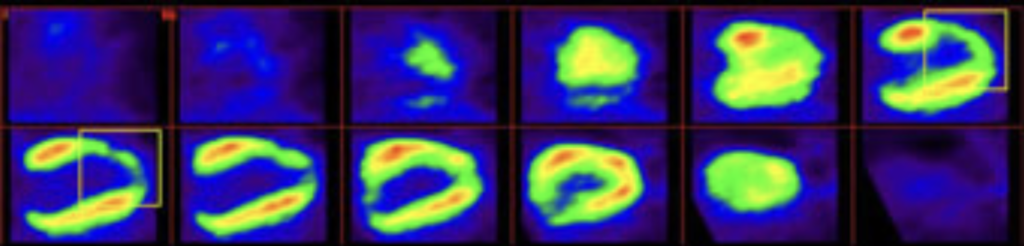
- However a severe heart attack (about 30% of the cases) leads to a chronic heart failure that weakens heart function and reduces life expectancy (survival rate of 50% at 5 years), despite the daily administration of conventional therapies whose actions are mainly symptomatic.
Post-AMI heart failure represents a crucial unmet medical need.
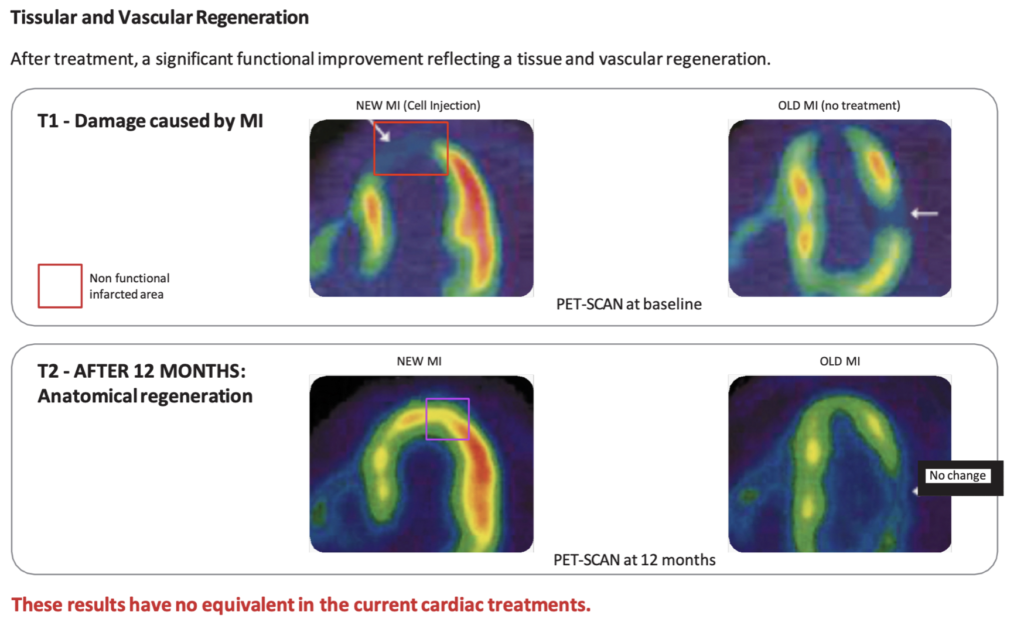
- Regenerative Medicine thus emerges as a new breakthrough model of medicine that provides a therapeutic solution to cure the disease, being capable to regenerate both structurally and functionally the damaged cardiac area.