ProtheraCytes®
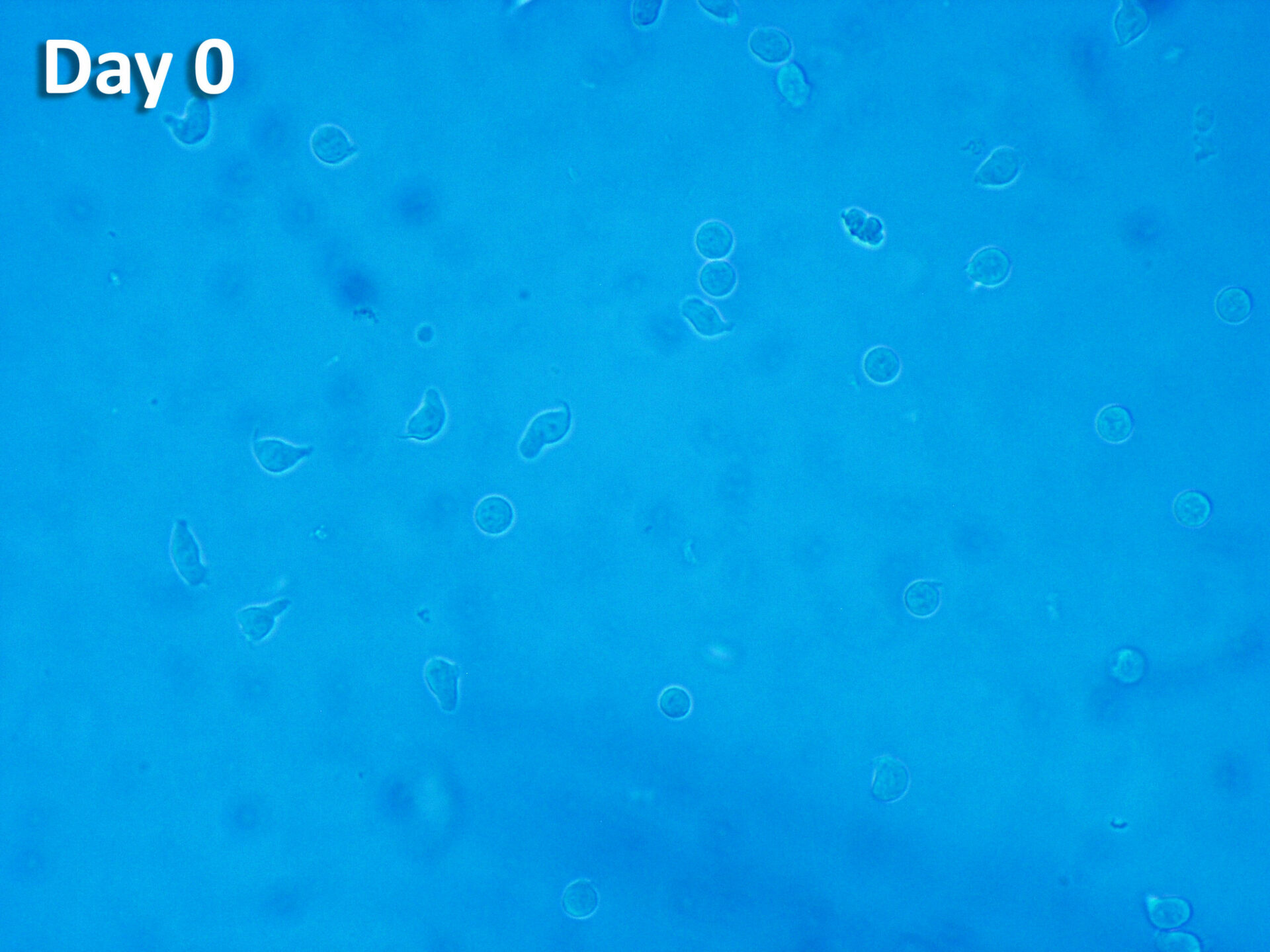
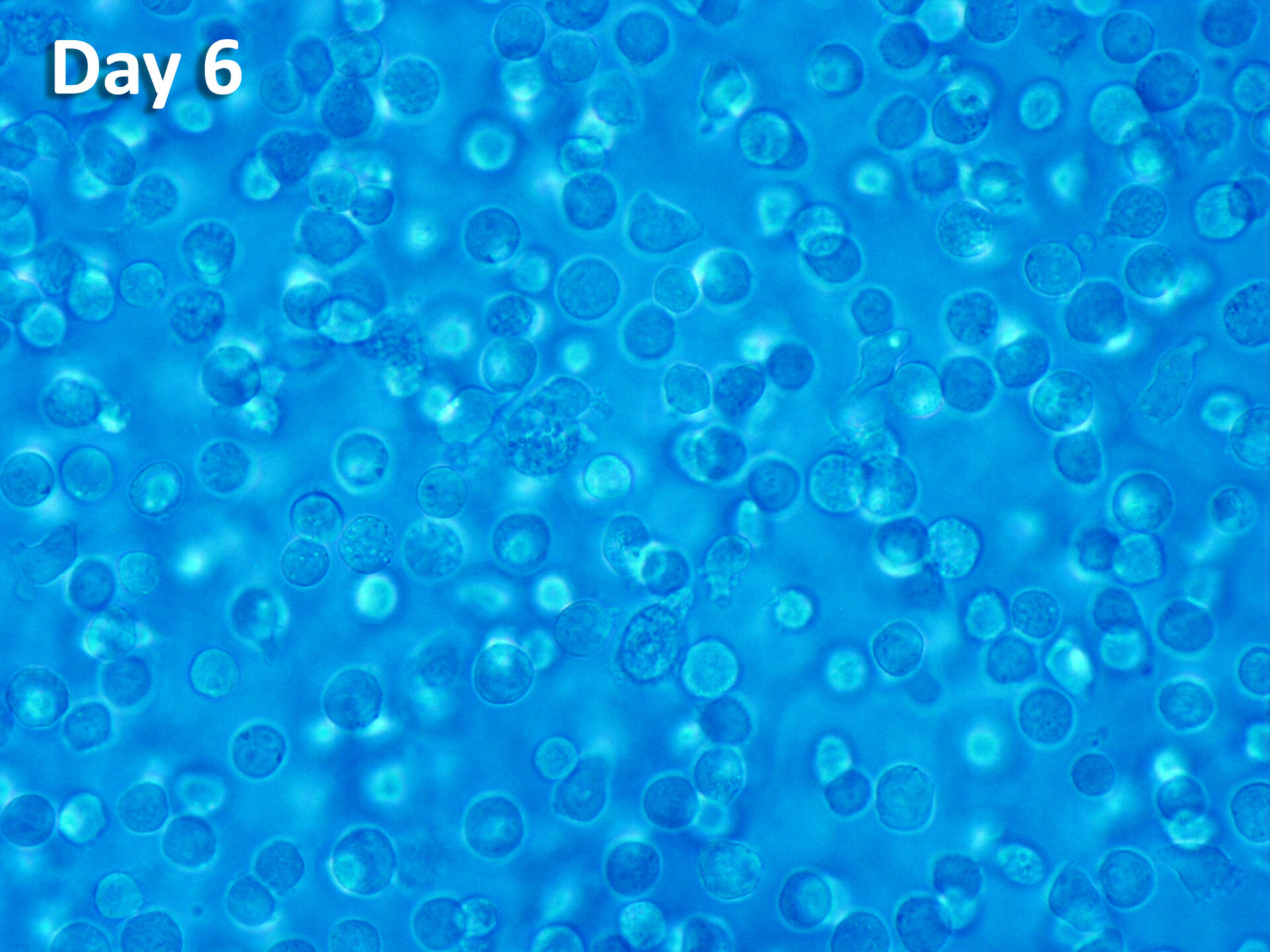
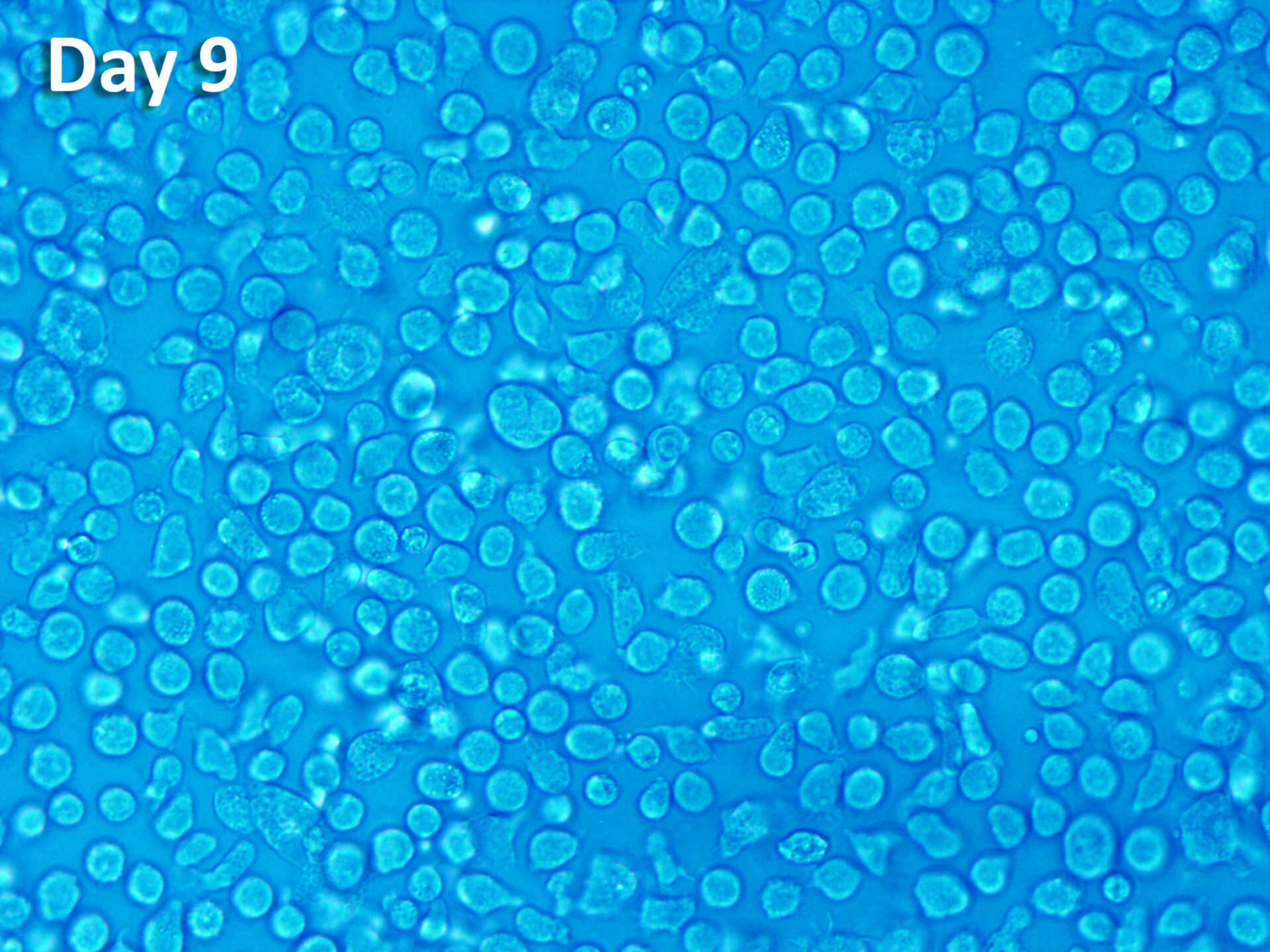
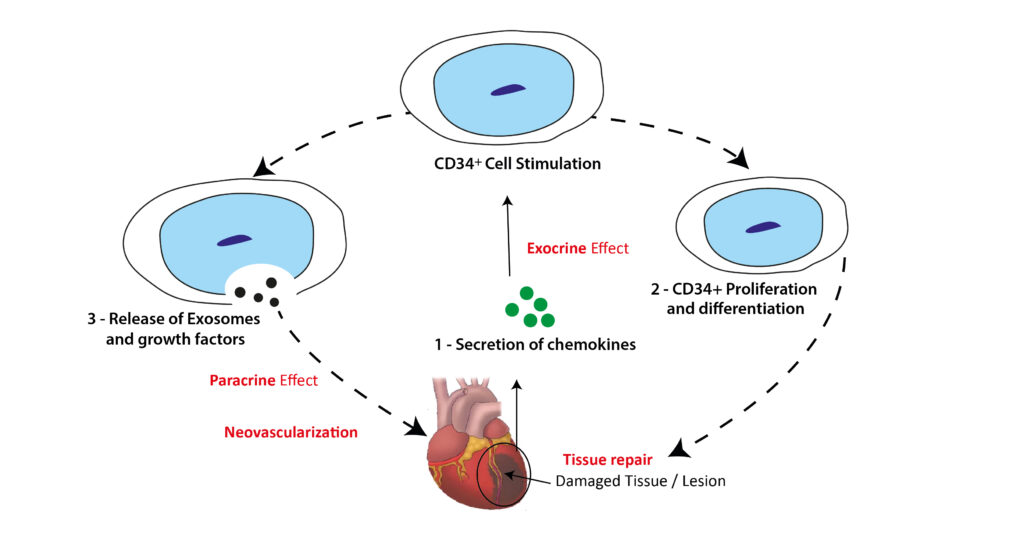
ProtheraCytes® is a fresh preparation of stem cells that has the potential for regeneration of various damaged tissues, including cardiac tissue. ProtheraCytes® is derived from CD34+ cells harvested from the peripheral blood of the patient after mobilization from the bone marrow, and the propietary expansion, purification, and formulation in a fresh cell suspension for injection.